Cancer in 3D: in-depth research to uncover its secrets
In 2012, 2.8 million people in the EU were diagnosed with cancer. It is the second most common cause of death in the Union – three out of 10 deaths for men, and two out of 10 deaths for women – a figure that is expected to rise due to the ageing European population. Dr Danijela Matic Vignjevic’s STARLIN project is using ERC funding to understand how normal cells become cancerous and spread.

Colorectal cancers, affecting the stomach and intestines, are among the most frequently occurring forms of cancer. With such a huge health impact, the EU is funding a great deal of research into cancer, but Dr Vignjevic’s research is unusual in that it focuses on the cell biology of the very early stages of cancerous development, rather than the later medical issues.
The entire lining of our intestine is renewed every week, thanks to cells dividing and multiplying, and then migrating towards the tips of the ‘villi’ – the finger-shaped protrusions that line the intestines and absorb nutrients from our food – before dying at the tips.
“Cancer may result when cells proliferate more than usual, or don’t die, or don’t migrate enough or normally,” she explains. “If we can understand how they migrate then we can better understand cancer.”
Looking deep into the cells
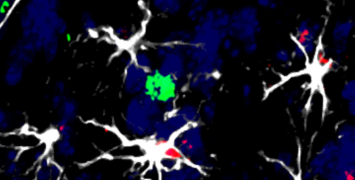
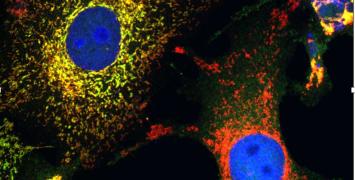
The basement membrane – part of the wall of the intestine – is a physical barrier that has to be penetrated for a tumour to spread to other tissues. The STARLIN project is taking a multidisciplinary approach, using new imaging tools to study the fundamental mechanisms of cell migration – both in cancerous invasion and in the normal function of the intestine. It is also looking at the interaction between cancer cells and the ‘stromal cells’ that make up the body’s connective tissue (such as the basement membrane).
“To investigate this properly we need to use primary material – samples from real patients – not cell lines grown in the laboratory,” says Dr Vignjevic. “So we work closely with surgeons in order to obtain biopsies of cells from different types of tumours and from different stages of development.”
Originally from Serbia, Dr Vignjevic is now based in Paris, France. “Having completed my PhD on cell migration in the US, I wanted to work on tissues in vivo – the Institut Curie is one of the best places to do this,” she says.
“There are two parts to the project,” she explains: “First, how do cells migrate, and how is the tissue renewed in normal gut tissue – is it passive or active? The second part is a detailed study of interactions between cancer and stromal cells during invasive migration. This is technically very challenging as it is very hard to image the interaction of cells in their microenvironment.”
New tools for new breakthroughs
“In order to study tumours we need 3D imaging systems,” Dr Vignjevic says. “We need to be able to make images of cells buried deep inside tissue.”
Thanks to an ERC grant, her research group has been able to buy and operate a ‘two-photon microscope’. This expensive equipment uses fluorescence to produce real-time images while inflicting less ‘phototoxicity’ damage to cells or tissue than comparative methods.
“This project bridges fields so we needed to build a team that includes cellular and molecular specialists, and cancer specialists, as well as physicists for the physical aspects of the biological environment,” she says. “Of the 10 people in our lab, seven or eight are working on this ERC project.”
The project, which only started last year and is scheduled to run until the end of 2017, has already made good progress in its study of how cancer cells break through the basement membrane to spread to other organs.
“We are now analysing these mechanisms,” the researcher continues. “And we are also making good progress in improving our understanding of cell migration, by imaging intestinal cells over three days from birth to death. It is technically challenging to culture tissue slices ex vivo in this way, but necessary in order to understand their cycle of proliferation, movement and death.”
The research may have applications in producing drugs that could target stromal cells, rather than cancer cells, in order to prevent metastasis. While such clinical uses are still a long way off, according to Dr Vignjevic: “We have four years ahead of us so we are optimistic about what we will be able to achieve.”