New landmark in epigenetics: understanding the silencing of the X-chromosome
While women inherit two X chromosomes, the expressions of one of them is shut down during embryonic development. Men have one X chromosome and one Y chromosome. The switching off of women’s second X chromosome is thought to compensate for the presence of only one X in males versus two in females, to balance for X-linked gene products between the sexes. X-chromosome inactivation is also one of the clearest examples of what epigenetic mechanisms do to our genetic material: the DNA of the genes on the X is still present but not actively expressed or needed. Prof. Edith Heard was awarded ERC grants to understand the intricate processes behind the phenomenon, with unexpected results that changed the way gene regulation is now looked at.
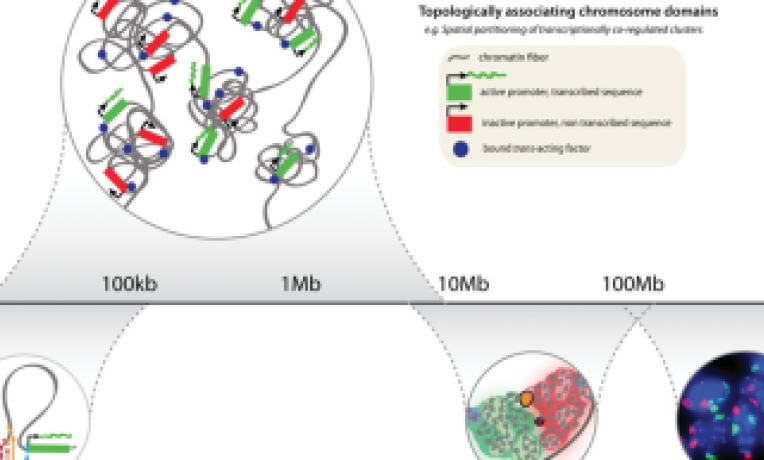

Prof. Heard’s team (working at the Institut Curie in the INSERM/CNRS unit of Genetics and Developmental Biology) uses X-chromosome inactivation as a model to understand how differences in gene expression states can be established and then perpetuated thanks to epigenetics. How do cells express or silence their genes, why is this sometimes reversible and can this process change in diseases such as cancer? How do cells recognise that they have two X chromosomes, not one, and trigger X inactivation?
One of the aims of Prof. Heard’s ERC project EpigenetiX was to study how the initiation of X inactivation is regulated. Thanks to the acquisition of a super-resolution (OMX) microscope, originally designed by her co-PI on this grant, Prof. John Sedat (University of California, San Francisco) and the use of novel molecular techniques (chromosome conformation capture, or 5C, in collaboration with
Job Dekker, UMass, USA), her team was able to investigate the way that DNA was folded within the X-inactivation centre, the master regulator of initiation of X inactivation. This provided unique insights into the regulatory potential and organisation of the Xic, as well as chromosome folding in general, as it led to the discovery of a new level of folding of the genome into Topologically Associating Domains (TADs) (*).
Her team also showed how TAD organisation of the genome is linked to biological function. The regulatory elements that affect the expression of a gene usually lie within the same TAD as the gene. Also genes that lie within the same TAD, show coordinated expression during development, while genes in separate TADs can show very different dynamics (*). This partitioning of the genome into
TADs could also explain why the alteration of DNA sequences can have an impact on a gene even at a long distance, within the same TAD (**) (***). Beyond the study of X inactivation, Prof. Heard’s team explored the extent to which monoallelic epigenetic regulation affects autosomal genes (****). These discoveries have had an impact on the study of gene regulation, opening unexplored lines of research in the field of epigenetics. For example by studying the role monoalleic gene expression during development and in the fully-developed body, the research opened new fields of investigation for the study of genetic diseases and cancer. The processes described could also advance regenerative medicine. During the term of her grant, Prof. Heard was elected Professor of the Collège de France (2012) and Fellow of the Royal Society (2013). With a second ERC Advanced Grant, awarded in 2015, she will use cutting edge approaches including CRISPR/Cas9 to decipher the molecular mechanisms behind X inactivation at the level of genes. Her results could uncover principles involved in healthy gene regulation and in the deregulations that occur as tumorous cells develop.