At the forefront of tissue engineering

By Alessandra Ferrari
From an early age, Liesbet Geris was fascinated by both biology and engineering. Although her interests did not align with pursuing a career in medicine, she aspired to bridge the gap between these two fields, pushing the frontiers of biological and biomedical research.
‘I already knew I was very much interested in biological and biomedical applications,’ Geris reflects, ‘but somehow becoming a doctor didn't really appeal to me.’ This passion set her on a path that would lead to cutting-edge research in regenerative medicine.
Her focus soon turned to bone regeneration, an area that allowed her to combine both engineering and biological aspects. ‘This is where I wanted to sit. Tissue engineering intentionally brings together engineering and biology, even in its name,’ she explains. This focus not only shaped her career but has also positioned her as an eminent figure in regenerative medicine, where she challenges conventional approaches to medical treatment through innovative solutions such as digital twins and scaffold design.
Researchers in regenerative medicine are continually breaking new ground, developing novel therapies to tackle diverse medical challenges. Liesbet Geris has been at the forefront of innovation with her work on osteochondral defects, cartilage regeneration, and digital twins. Her research aims to revolutionise medical treatments and improve patient outcomes. Here, we delve into the complexities of her work and its potential impact.
The blueprint for regeneration
At the heart of Geris's research is a ‘skeleton’ blueprint, a key outcome of her completed ERC-funded INSITE project. This model serves as a foundational map, helping researchers understand how specific treatments affect the biological pathways of individual cells. ’We use it as a blueprint to see how the treatments that we apply guide certain cells along a particular biological path, and how closely these cells still resemble the original cells from the body,’ she explains.
Comparable to a skeletal cell atlas, the model enables scientists to project cells onto it and monitor whether they have reached their intended target- a journey she compares to sending cells from one location to another. ‘For example, if I gave them a treatment thinking I would send them to, let’s say, Brussels, and now I put them on the map. Are they actually in Brussels, or are they in Antwerp instead?’ Geris humorously notes.
Cell differentiation
The process of cell differentiation is central in Geris's research. Starting from a specific cell type, scientists guide it through a series of transformations until it reaches its target state. Her team focuses primarily on autologous patients’ cells, including periosteal cells and cartilage-derived cells. By refining how these cells are manipulated, Geris and her team hope to enhance the effectiveness and applicability of cell-based treatments.
’You start from a certain cell type to end up with a different, differentiated cell type,’ Geris explains, highlighting the careful, step-by-step journey that turns ordinary cells into functional components of living tissue.
Geris is also advancing the concept of the ‘digital twin’ in the context of osteochondral defects, cartilage, and tissue engineering. By combining various tools and models, researchers can create virtual versions of targeted tissue and use these to test different treatment strategies, improving outcomes before any clinical intervention.
About the ongoing ERC Consolidator Grant project INSTant CARMA, Geris explains, ‘we are using all the tools we have developed in the previous ERC project INSITE and adapting them to a different model. It requires different inputs, but we're recycling some of existing tools and enhancing many others, now focusing not on the large bone defects, but on osteochondral defects.’ The project combines multiple technologies to enhance tissue engineering outcomes, aiming for more effective and personalised treatments.
From lab to clinic
Geris's work extends far beyond theoretical modelling. Her team strives to bridge the gap between laboratory-based research and clinical application. ’We're now working with the GMP (Good Manufacturing Practices) facility in another project, which focuses on the translational aspects -creating cartilage patches that could be used in humans in as early as 2026,’ she says.
Osteochondral defects, often occur in osteoarthritis, are a key focus. As these conditions currently lack effective treatments, the work has the potential to relieve pain and restore mobility for many patients.
A significant goal of Geris's work is to develop living implants, a complex process that requires rigorous testing in an animal model before advancing to human trials. She emphasises the crucial role of the biological models her team has developed to support this work. Collaborations also play a crucial role in bringing these innovations to life. One such partnership is with CerHUM, a spin-off that produces calcium phosphate scaffolds already in clinical use. ‘They are creating implants with the microstructure we designed that actually go into the human body,’ she explains.
Insights into the immune system
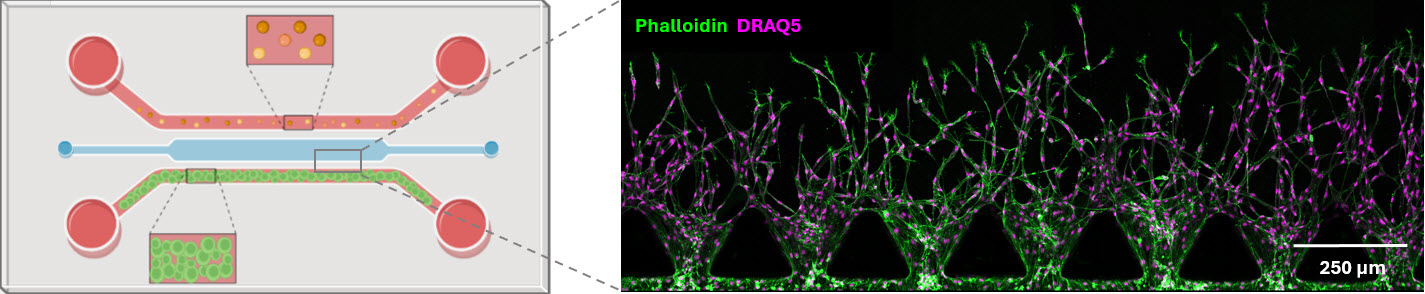
Geris also explores how the immune system shapes bone regeneration. Her team combines omics data with microfluidic chips to study the interplay between tissue mechanics, inflammation, and cell behaviour.
’In INSITE, we also started using microfluidic chips to be able to look at the interaction between, for instance, tissue mechanics and the immune system, or tissue mechanics and inflammation processes’, she says.
This approach provides a more detailed understanding of the biological processes that underpin regenerative solutions, which is crucial for improving their long-term success.
Despite the promise of her work, Geris acknowledges the complexities and challenges that come with translating research into real-world applications. From regulatory hurdles to quality control and economic viability, the path from lab to clinic is rarely straightforward.
Yet some of these challenges also create new opportunities. For example, Geris and her team coordinated an ecosystem-wide effort to write a roadmap for realising the vision of the Virtual Human Twin, accelerating the development and uptake of digital twins in health and care in Europe. The document now also serves as a valuable onboarding tool for new students and collaborators, offering a comprehensive overview of the field and its complexities.
Liesbet Geris's pioneering work captures the transformative potential of regenerative medicine. By leveraging cutting-edge technology and integrating insights across disciplines, she and her team are developing solutions that could redefine how we treat complex conditions. While many questions remain, their research lays the foundations for a future in which healing is faster, smarter, and more personalised, offering new hope to patients worldwide.
Biography
Liesbet Geris is Professor of Biomechanics and Computational Tissue Engineering at the University of Liège and KU Leuven in Belgium. Her research focuses on developing enabling technologies - both in silico and in vitro - for skeletal tissue engineering. She is the scientific coordinator of Prometheus, a musculoskeletal tissue engineering platform, and has received several young investigator and research awards. As Executive Director of the Virtual Physiological Human Institute, she promotes the use of in silico modelling in heablthcare through collaboration with clinicians, the European Commission and Parliament, regulatory agencies, and patient communities.






